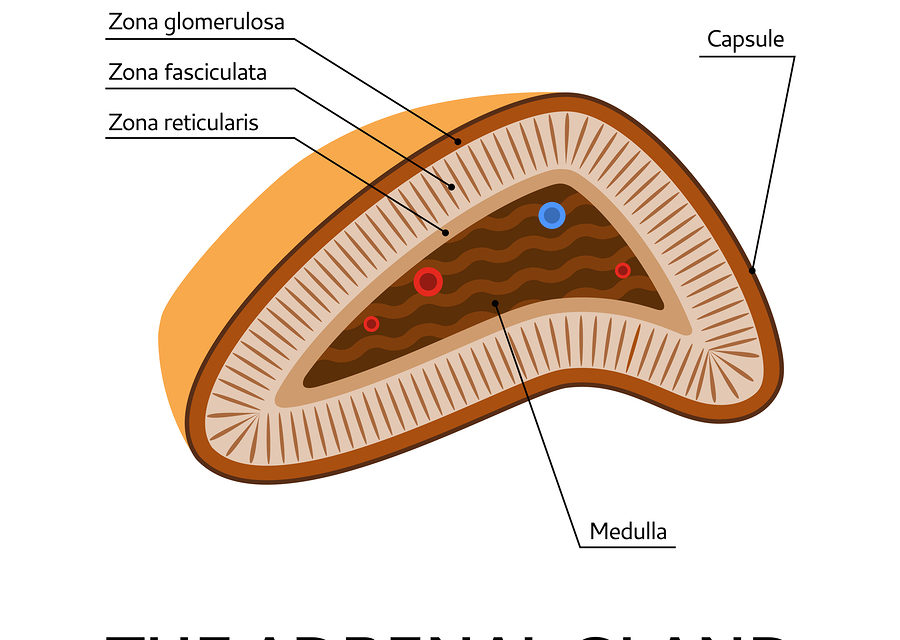
The use of topical steroids for skin problems may suppress the adrenal glands. According to an article in Family Practice News (January 1, 2004:69), the Food and Drug Administration had recommended that a black box warning be placed on topical steroids. A “black box” warning appears on prescription drugs that may cause serious adverse effects. The warning usually has a black border surrounding the text of the warning—hence the name. When medical studies indicate that the drug carries a significant risk of serious side-effects, the U.S. Food and Drug Administration (FDA) can require a pharmaceutical company to place a black box warning on the labeling of a prescription drug, or in literature describing it. It is the strongest warning that the FDA requires.
Use of topical steroids may cause asymptomatic, secondary adrenal suppression when used to treat atopic dermatitis in children. The problem is asymptomatic until the child experiences trauma or infection which may trigger acute adrenal suppression. The symptoms of acute adrenal insufficiency include low blood pressure, weakness, fatigue, nausea, and vomiting are often mistaken for something else. Topical steroids are often used in very young children to treat atopic dermatitis—frequently under the age of six months.